
Reports of adverse reactions to spot-on pesticides increased 53% from 2007-2008. That’s a big increase and the EPA launched a study. Spot-on pesticides are those flea & tick treatments you apply between pet’s shoulder blades or down their spine like Defend ExSpot, Sergent’s Squeeze-On, Hartz Flea & Tick Drops, Bio Spot, Advantage, Frontline, etc. Spot-on products fall under the purview of the EPA instead of the FDA because they are a pesticide, not a medication. A scary ramification of EPA oversight instead of FDA oversight is that there’s been no post-market tracking of the products. That means once they’re registered by their manufacturer, these spot-on pesticides haven’t been monitored. Not good, but things are changing.
The EPA’s review of data on spot-on pesticides found most adverse reactions were minor, such as skin irritations that resolved quickly, however, they’re were disabilities & death. In cats, the problem was usually inappropriate application of dog products, which contained ingredients dangerous for cats or were too large a dose. In dogs, small & toy breeds had more adverse reactions than larger dogs probably due to too high a dose. Here’s what the EPA plans to do:
Improving labeling as needed to insure product:
- is very clearly identified as for cats or for dogs only
- lists safety precautions, such as keeping dogs apart from cats immediately after product is applied
- lists possible reactions
Safety changes:
- changing dosage by narrowing weight ranges (a current weight range for one brand is 23-44lbs, which means a 23lbs. dog is getting almost twice as much of the active ingredient per pound as a 44lbs. dog at the high end of the weight range)
- determining safety of inert ingredients which can enhance absorption through skin, cause skin sensitization, be toxic to cats
- improving testing requirements to examine differences between dogs, long-term exposure, effects of exposure orally from grooming
- appropriate testing–beagles are usually used in testing and this breed isn’t sensitive to spot-on pesticides
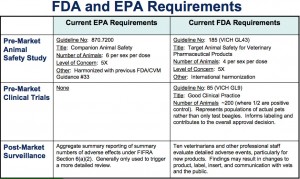
- bring EPA’s data requirements inline with FDA’s data requirements (click on the chart below to see how they compare at this time)
EPA’s Safety Tips:
- Consult your veterinarian about the best way to to protect your pets from fleas and ticks and whether pesticides are even needed.
- Use extra care before use on weak, aged, medicated, sick, pregnant, or nursing pets, or on pets that have previously shown signs of sensitivity to pesticide products.
- If you use a spot-on product or any other pesticide on your pet, carefully read and follow the product label.
- Use flea and tick control products only on the animal specified by the product label – for example, dog products for dogs only and cat products for cats only.
- Follow any label prohibitions against use on weak, aged, medicated, sick, pregnant, or nursing pets, or on pets that have previously shown sensitivity to pesticide products. Apply only the amount indicated for the size of the animal being treated.
- Do not apply to kittens or puppies unless the product label specifically allows this treatment. Pay attention to the age restrictions for puppies and kittens on the label.
- Monitor your pet for side effects or signs of sensitivity after applying the product, particularly when using the product on your pet for the first time. Do not apply spot-ons to pets known to be sensitive to pesticide products.
- Keep the package with the product container (such as individual applicator tubes). Also keep the package after treatment in case adverse effects occur.
- If your pet experiences an adverse reaction, immediately bathe the pet with mild soap and rinse with large amounts of water.
For more information check out:


